Abstract
The term “acetal resins” commonly denotes the family of homopolymers and copolymers whose main chains are completely or essentially composed of repeating oxymethylene units 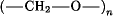 . The polymers are derived chiefly from formaldehyde or methanal, either directly or through its cyclic trimer, trioxane or 1,3,5-trioxacyclohexane. The many commercially attractive properties of acetal resins are due in large part to the inherent high crystallinity of the base polymers, which contributes significantly to their excellent resistance to most chemicals, including many organic solvents. Stiffness, creep resistance, fatigue resistance, and excellent lubricity are properties of acetal resins which have contributed significantly to their commercial success. Acetal resins are generally stable in mildly alkaline environments. Properly end-capped acetal resins are relatively thermally stable. Formaldehyde polymerizes by both anionic and cationic mechanisms. Copolymerization of trioxane with cyclic ethers or formals is accomplished with cationic initiators. All acetal resins contain various stabilizers introduced by the supplier. Acetal resins are most commonly fabricated by injection molding. They are used in conveying devices, gears, automotive parts, and household appliances.
. The polymers are derived chiefly from formaldehyde or methanal, either directly or through its cyclic trimer, trioxane or 1,3,5-trioxacyclohexane. The many commercially attractive properties of acetal resins are due in large part to the inherent high crystallinity of the base polymers, which contributes significantly to their excellent resistance to most chemicals, including many organic solvents. Stiffness, creep resistance, fatigue resistance, and excellent lubricity are properties of acetal resins which have contributed significantly to their commercial success. Acetal resins are generally stable in mildly alkaline environments. Properly end-capped acetal resins are relatively thermally stable. Formaldehyde polymerizes by both anionic and cationic mechanisms. Copolymerization of trioxane with cyclic ethers or formals is accomplished with cationic initiators. All acetal resins contain various stabilizers introduced by the supplier. Acetal resins are most commonly fabricated by injection molding. They are used in conveying devices, gears, automotive parts, and household appliances.
Keywords: Acetal resins; Homopolymer; Copolymer; Finishing; Fabrication; Resin grades; Scrap; Solvent resistance; Formaldehyde
