Abstract
The activated aluminas comprise a series of nonequilibrium forms of partially hydroxylated aluminum oxide, Al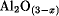 (OH)
(OH)
Keywords: aluminum oxide; alumina; activated; aluminum hydroxide; catalysts; membranes; adsorbents; bachmite
If you are seeing this message, you may be experiencing temporary network problems. Please wait a few minutes and refresh the page. If the problem persists, you may wish to report it to your local Network Manager.
It is also possible that your web browser is not configured or not able to display style sheets. In this case, although the visual presentation will be degraded, the site should continue to be functional. We recommend using the latest version of Microsoft or Mozilla web browser to help minimise these problems.
 | |||||
| |||||
AbstractThe activated aluminas comprise a series of nonequilibrium forms of partially hydroxylated aluminum oxide, Al Keywords: aluminum oxide; alumina; activated; aluminum hydroxide; catalysts; membranes; adsorbents; bachmite | |||||