Abstract
Among industrial users of sodium aluminate are producers of paper, paint pigments, silica, alumina or alumina-based catalysts, dishwasher detergents, molecular sieves, concrete, antacids, and others. Sodium aluminate is used in removal of phosphates from municipal and industrial waste waters and for clarification of industrial process and potable water. Commercial sodium aluminate products are available as liquids, and to a lesser degree, in solid form. The formula of anhydrous sodium aluminate is variously given as NaAlO2 (aluminum sodium oxide), 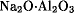 , or Na2Al2O4. Commercial sodium aluminates are not accurately represented by these formulas because the products contain more than the stoichiometric amount of sodium oxide, Na2O. The amount of excess caustic in commercial products is indicated by ratios of Na2O/Al2O3. Commercial quantities of sodium aluminate are made from hydrated alumina, in the form of aluminum hydroxy oxide, AlO(OH), or aluminum hydroxide, Al(OH)3, a product of the Bayer process which is used to refine bauxite, the principal aluminum ore. In addition to treatment of industrial and municipal water supplies, sodium aluminate is used in large quantities in papermaking where it improves sizing, filler retention, and pitch deposition. Sodium aluminate is also widely used in the preparation of alumina-based catalysts.
, or Na2Al2O4. Commercial sodium aluminates are not accurately represented by these formulas because the products contain more than the stoichiometric amount of sodium oxide, Na2O. The amount of excess caustic in commercial products is indicated by ratios of Na2O/Al2O3. Commercial quantities of sodium aluminate are made from hydrated alumina, in the form of aluminum hydroxy oxide, AlO(OH), or aluminum hydroxide, Al(OH)3, a product of the Bayer process which is used to refine bauxite, the principal aluminum ore. In addition to treatment of industrial and municipal water supplies, sodium aluminate is used in large quantities in papermaking where it improves sizing, filler retention, and pitch deposition. Sodium aluminate is also widely used in the preparation of alumina-based catalysts.
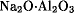 , or Na
, or Na