Abstract
In 1976 scientists at the Merck Corporation discovered a complex of eight closely related natural products, subsequently named avermectins A1a through B2b, in a culture of Streptomyces avermitilis MA-4680 (NRRL8165) originating from a soil sample collected at Kawana, Ito City, Japan and isolated by the Kitasato Institute. Designated A1a (C49H74O14), A1b (C48H72O14), A2a (C49H76O15), A2b (C48H74O15), B1a (C48H72O14), B1b (C47H70O14), B2a (C48H74O15), and B2b (C47H72O15), they are among the most potent anthelmintic, insecticidal, and acaricidal compounds known. Two avermectins, abamectin (the avermectin B1) and ivermectin, which is saturated at C-22–C-23, have been commercialized to date. Avermectin B1 is the most effective of the avermectin family of natural products against agriculturally important insects and mites. Ivermectin is active against two significant phyla of animal parasite: the Nemathelminthes or nematodes (roundworms) and the Arthropoda (insects, ticks, and mites). Avermectins are highly lipophilic substances and dissolve in most organic solvents. Avermectin B1 and ivermectin both contain two secondary and one tertiary hydroxy group. The two secondary alcohols are readily acetylated with acetic anhydride in pyridine. Avermectin B1 and ivermectin have two secondary hydroxy groups susceptible to oxidation to the corresponding ketones. Ivermectin (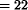 ,23—dihydroavermectin B1) is obtained by catalytic reduction of avermectin B1. Methylation of avermectins B1 and B2 leads to the corresponding derivatives of the A series. The worldwide acceptance of ivermectin in livestock production and health care of companion animals has made it the largest selling animal health drug. Abamectin is in commercial use as an agricultural pesticide and its applications are continuing to expand.
,23—dihydroavermectin B1) is obtained by catalytic reduction of avermectin B1. Methylation of avermectins B1 and B2 leads to the corresponding derivatives of the A series. The worldwide acceptance of ivermectin in livestock production and health care of companion animals has made it the largest selling animal health drug. Abamectin is in commercial use as an agricultural pesticide and its applications are continuing to expand.
Keywords: Avermectins; Abamectin; Ivermectin; Mites; Insects; Monosaccharides; Aglycones; Biosynthesis; Merck Corporation; Insecticides; Acaricides; Anthelmintic agents
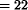 ,23—dihydroavermectin B
,23—dihydroavermectin B