Abstract
The term azo dyes is applied to those synthetic organic colorants that are characterized by the presence of the chromophoric azo group (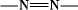 ). This divalent group is attached to sp
). This divalent group is attached to sp
Initially, the focus of attention of mechanistic work is on the oxidation of dyes by typical detergent bleaches, which occurs by oxygen atom transfer. This is followed by an outline of electron-transfer mechanisms and radical mechanisms or by metal catalysis, involving high oxidation state species. Finally, attention is directed towards understanding photofading mechanisms which, although more complex, is facilitated by the foregoing discussion.
Of all classes of dyestuffs, azo dyes have attained the widest range of usage because variations in chemical structure are readily synthesized and methods of application are generally not complex. There are azo dyes for dyeing all natural substrates such as cotton, paper, silk, leather, and wool; and there are azo dyes for synthetics such as polyamides, polyesters, acrylics, polyolefins, viscose rayon, and cellulose acetate; for the coloring of paints, varnishes, plastics, printing inks, rubber, foods, drugs, and cosmetics; for staining polished and absorbed surfaces; and for use in diazo printing and color photography. The shades of azo dyes cover the whole spectrum. Commercial acid dyes contain one or more sulfonate groups, thereby providing solubility in aqueous media. There are three general classifications of acid dyes: acid dyes that dye directly from the dyebath, mordant dyes that are capable of forming metallic lakes on the fiber when aftertreated with metallic salts, and premetallized dyes. Direct dyes are defined as anionic dyes substantive to cellulosic fibers (cotton, viscose, etc), when applied from an aqueous bath containing an electrolyte. Direct dyes are one of the most versatile classes of dyestuff. In worldwide usage for cellulosic textiles, direct dyes are the second largest class of dyestuff. Direct yellows and oranges, direct reds, and direct blues are prominent direct dyes. Direct violets and greens are small-volume products. Azoic dyes (known also as ice colors and ingrain colors) are water-insoluble azo pigments. Disperse dyes are coloring substances having very low aqueous solubility which are applied to hydrophobic fibers from an aqueous system in which the dye is present in a highly dispersed state. The sharp increase in the importance of disperse dyes in the 1970s and 1980s can be attributed directly to the emergence of polyester and nylon as the principal synthetic fibers. Azo and anthraquinone compounds comprise the two principal structural types which are used as disperse dyes. Manufacturing procedures for producing dye dispersions are generally not disclosed. The principal dispersants in use include long-chain alkyl sulfates, alkaryl sulfonates, fatty amine–ethylene oxide condensates, and others. Disperse dyes are classified as high energy or low energy types. The use of higher dyeing temperatures for polyester fibers has made possible the use of dyes of higher molecular weight, the so-called high energy dyes. A use for disperse dyes which has undergone rapid growth since 1970 is in inks for the heat-transfer printing of polyester. The oil-soluble, water-insoluble, azo dyes dissolve in oils, fats, waxes, etc. Generally, yellow, orange, red, and brown oil colors are azo structures and greens, blues, and violets are primarily anthraquinones. Spirit-soluble azo dyes dissolve in polar solvents, such as alcohol and acetone, and find application in the coloring of lacquers, plastics, printing inks, and ball-point pen inks. Basic dyes of the azo class are the simplest and oldest known synthetic dyes. Current cationic dyes are used for modified acrylics, modified nylons, modified polyesters, leather, unbleached papers, and inks. An important application is for conversion into pigments. Organic pigments are an important class of organic colorants. Expanding areas of usage include the mass coloration of synthetic fibers and textile printing in the textile field, and in the nontextile area, plastics. A pigment is insoluble in the medium in which it is used. The physical properties of pigments are of great significance since the coloring process does not involve solution of the colorant. Azo pigments can be grouped as metal toners, metal chelates, and metal-free azo pigments.
Keywords: classification; azo dyes; dye degradation; acid dyes; metal complexes; direct dyes; napthal dyes; coupling components; fast color; disperse dyes; heterocyclic disperse dyes; oil soluble dyes; spirit soluble azo dyes; cationic dyes; pigments
