Abstract
Barite, natural barium sulfate, BaSO4, commonly known as barytes, and sometimes as heavy spar, till, or cawk, occurs in many geological environments in sedimentary, igneous, and metamorphic rocks. Most commercial sources are replacement deposits in limestone, dolomitic sandstone, and shales. Barite is widely distributed, with minable deposits in many countries. Barite is a moderately soft crystalline mineral. Residual barite is usually mined by open-pit methods. Most of the world's barite production is used as a weighting agent for the muds circulated in rotary drilling of oil and gas wells. Finely ground barite is used as a filler or extender in paint especially in automotive undercoats. It is also used as a filler in plastics and rubber products. Barium acetate, is used in printing fabrics, lubricating grease, and as a catalyst for organic reactions. Reported uses of barium bromide, BaBr2, include fabrication of phosphors and as a crystallization nucleating agent to control supercooling of CaBr2 solutions. Most barium compounds are prepared from reactions of barium carbonate, which is commercially manufactured by the “black ash” process from barite and coke in a process identical to that for strontium carbonate production. Precipitated or synthetic barium carbonate is the most commercially important of all the barium chemicals except for barite. Several different grades of barium carbonate are manufactured to fit the specific needs of a wide variety of applications: very fine, highly reactive grades are made for the chemical industry; coarser and more readily handleable grades are mainly supplied to the glass industry. Barium chloride, is used in heat treating baths because of the eutectic mixtures it readily forms with other chlorides and to set up porcelain enamels for sheet steel. Barium 2-ethylhexanoate, also known as barium octanoate or barium octoate, is usually used in synergistic combination with cadmium or zinc organic salts as a thermal stabilizer for PVC. Barium fluoride is used commercially in combination with other fluorides for arc welding, electrode fluxes, and in the manufacture of fluorophosphate glasses and infrared transmitting windows. Properties and preparation methods are given. Boron trifluoride is widely used as an acid catalyst, especially for the production of petroleum and polymer products, such as in the manufacture of synthetic lubricants and in the preparation of hydrocarbon resins. Boron trifluoride is also finding increased use as a chemical reagent in the synthesis of boron-containing materials of various types. Barium hydroxide is the strongest base and has the greatest water-solubility of the alkaline-earth elements. It is used in the manufacture of barium greases and plastic stabilizers such as barium 2-ethylhexanoate, in papermaking, in sealing compositions, vulcanization accelerators, water purification, pigment dispersion, in a formula for self-extinguishing polyurethane foams, and in the protection of objects made of limestone from deterioration. Barium iodide dihydrate, is useful in making other iodides. Barium metaborate monohydrate, is used in flame retardant plastic formulations as a synergist for phosphorus or halogen compounds. Barium nitrate, which occurs as colorless crystals, is used in pyrotechnic green flares, tracer bullets, primers, and in detonators. Barium nitrate, has been used in diazotization reactions. Barium oxide, occurs as colorless cubic or hexagonal crystals. BaO is used to impart improved strength to porcelain, as a solid base catalyst, in specialty cements, and for drying gases. Reported uses of BaO2 include in the cathodes of fluorescent lamps, and as a drying agent for lithographic inks. Barium sodium niobium oxide, finds application for its dielectric, piezoelectric, nonlinear crystal and electrooptic properties. It has been used in conjunction with lasers for second harmonic generation and frequency doubling. Barium sulfate, occurs as colorless rhombic crystals. Because of its extreme insolubility BaSO4 is not toxic. In medicine, barium sulfate is widely used as an x-ray contrast medium. It is also used in photographic papers and as a filler for plastics. Barium sulfide, is used in the manufacture of lithophone, useful as a white pigment in paints. The basic crystal structure of barium titanate leads, to unique, outstanding dielectric properties. Barium titanate has widespread use in the electronics industry, eg, for miniature capacitors, and also in numerous sonic and ultrasonic devices. Yttrium–barium–copper oxide, is a high Tc material which has been found to be fully superconductive at temperatures above 90 K, a temperature that can be maintained during practical operation. Ultrapure powders of yttrium–barium–copper oxide that are sinterable into single-phase superconducting material at low temperatures are required creating a worldwide interest in high purity barium chemicals. The average adult human body contains 22 mg Ba, of which 93% is present in bone. The remainder is widely distributed throughout the soft tissues of the body in very low concentrations. Barium constitutes about 0.04% of the earth's crust. Agricultural soils contain 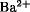 in the range of several micrograms per gram. The Environmental Protection Agency, under the Safe Drinking Water Act, has set a limit for barium of 1 mg/L for municipal waters in the United States. The toxicity of barium compounds depends on solubility.
in the range of several micrograms per gram. The Environmental Protection Agency, under the Safe Drinking Water Act, has set a limit for barium of 1 mg/L for municipal waters in the United States. The toxicity of barium compounds depends on solubility.
Keywords: Barium Compounds; Barium Sulfate; Barite; Barium Carboxate; Weighting Agents; Gas Weels; Die weck; Barium flurides
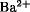 in the range of several micrograms per gram. The Environmental Protection Agency, under the Safe Drinking Water Act, has set a limit for barium of 1 mg/L for municipal waters in the United States. The toxicity of barium compounds depends on solubility.
in the range of several micrograms per gram. The Environmental Protection Agency, under the Safe Drinking Water Act, has set a limit for barium of 1 mg/L for municipal waters in the United States. The toxicity of barium compounds depends on solubility.