Abstract
Barrier polymers are used for many packaging and protective applications. As barriers they separate a system, such as an article of food or an electronic component, from an environment. Barrier polymers limit movement of substances, called permeants. The movement can be through the polymer or, in some cases, merely into the polymer. After crossing the barrier polymer, the permeant moves to the polymer surface, desorbs, and moves away. Permeant movement is a physical process that has both a thermodynamic and a kinetic component. For polymers without special surface treatments, the thermodynamic contribution is in the solution step. The permeant partitions between the environment and the polymer according to thermodynamic rules of solution. The kinetic contribution is in the diffusion. The net rate of movement is dependent on the speed of permeant movement and the availability of new vacancies in the polymer. The traditional definition of a barrier polymer required an oxygen permeability < 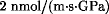 [originally, <
[originally, < .gif) ] at room temperature. Poly(ethylene terephthalate) (PET), with an oxygen permeability of
] at room temperature. Poly(ethylene terephthalate) (PET), with an oxygen permeability of .gif) , is not considered a barrier polymer by the old definition; however, it is an adequate barrier polymer for holding carbon dioxide in a 2-L bottle for carbonated soft drinks. Many months are required to lose enough carbon dioxide (15% of initial) to be objectionable. The polymers that are good barriers to permanent gases, especially oxygen, have important commercial significance. Vinylidene chloride copolymers are available as resins for extrusion, latices for coating, and resins for solvent coating. Vinylidene chloride copolymers are marketed under a variety of trade names. Saran is a trademark of The Dow Chemical Company for vinylidene chloride copolymers. Other trade names include Daran (W.R. Grace), Amsco Res (Union Oil), and Serfene (Morton Chemical) in the United States; and Haloflex (Imperial Chemical Industries, Ltd.), Diofan (BASF), Ixan (Solvay and Cie SA), and Polyidene (Scott-Bader) in Europe. Hydrolyzed ethylene–vinyl acetate copolymers, commonly known as ethylene–vinyl alcohol (EVOH) copolymers, are usually used as extrusion resins, although some may be used in solvent-coating applications. Copolymers of acrylonitrile are used in extrusion and molding applications. Commercially important comonomers for barrier applications include styrene and methyl acrylate. Polyamide polymers can provide a good-to-moderate barrier-to-permeation by permanent gases. Two often-used polymers have adequate properties for some applications. Poly(ethylene terephthalate) is used to make films and bottles. Poly(vinyl chloride) (PVC) is a moderate barrier-to-permanent gases. Plasticized (PVC) is used as a household wrapping film. In regard to water–vapor transmission rate (WVTR) values, those polymers that are good oxygen barriers are often poor water–vapor barriers and vice versa. Polymer molecules without dipole–dipole interactions, such as polyolefins, dissolve very little water and have low WVTR and permeability values. The permeation of flavor, aroma, and solvent molecules in polymers follows the same physics as the permeation of small molecules, but with two significant differences. For these larger molecules, the diffusion coefficients are much lower and the solubility coefficients are much higher. Furthermore, the large solubility coefficient can lead to enough sorption of the large molecule so that plasticization occurs in the polymer, which can increase the diffusion coefficient. Generally, vinylidene chloride copolymers and glassy polymers such as polyamides and EVOH are good barriers to flavor and aroma permeation, whereas the polyolefins are poor barriers. Several physical factors can affect the barrier properties of a polymer. These include temperature, humidity, orientation, and cross-linking. Typically, the permeability increases 5–10% for every increase of 1°C. When a polymer equilibrates with a humid environment, it absorbs water. This can plasticize the polymer and increase the permeability. The effect of orientation on the permeability of polymers is difficult to assess; diffusion in some polymers is unaffected by orientation; in others, increases or decreases are observed. Cross-linking has been shown in a few cases to decrease the diffusion coefficient. Reasonable prediction can be made of the permeabilities of low molecular weight gases such as oxygen, nitrogen, and carbon dioxide in many polymers. The diffusion coefficients are not complicated by the shape of the permeant, and the solubility coefficients of each of these molecules do not vary much from polymer to polymer. Reasonable predictions of the permeabilities of larger molecules such as flavors, aromas, and solvents are not easily made. The diffusion coefficients are complicated by the shape of the permeant, and the solubility coefficients for a specific permeant can vary widely from polymer to polymer. The permachor method is an empirical method for predicting the permeabilities of oxygen, nitrogen, and carbon dioxide in polymers. In this method, a numerical value is assigned to each constituent part of the polymer. An average number is derived for the polymer, and a simple equation converts the value into a permeability. The model has been modified to liquid permeation with some success. For larger molecules, independent predictions of the diffusion coefficients and the solubility coefficients are required. Predicting the diffusion coefficient for a permeant in a polymer requires knowing one other diffusion coefficient in the polymer. The solubility coefficients are more difficult to predict. Although advances are being made, the best method is probably to use a few known solubility coefficients in the polymer to predict others. Measuring the barrier properties of polymers is important for several reasons. The effects of formulation or process changes need to be known, new polymers need to be evaluated, data are needed for a new application before a large investment has been made, and fabricated products need to have performance verified. Two methods of measuring WVTR are commonly used. The newer method uses a Permatran-W (Modern Controls, Inc.). The other method is the American Society for Testing and Materials (ASTM) cup method. Measuring the permeation of carbon dioxide occurs far less often than measuring the permeation of oxygen or water. The simplest method uses the Permatran-C instrument (Modern Controls, Inc.). Many methods are used to characterize the transport of flavor, aroma, and solvent molecules in polymers. Each has some value, and no one method is suitable for all situations. Any experiment should obtain the permeability, the diffusion coefficient, and the solubility coefficient. The primary application for barrier polymers is food and beverage packaging. Barrier polymers are also used for packaging medical products, agricultural products, cosmetics, and electronic components and in moldings, pipe, and tubing. The use of safe materials is vital for barrier applications, particularly for food, medical, and cosmetics packaging. Suppliers of specific barrier polymers can provide the necessary details to ensure safe processing and use of barrier polymers.
, is not considered a barrier polymer by the old definition; however, it is an adequate barrier polymer for holding carbon dioxide in a 2-L bottle for carbonated soft drinks. Many months are required to lose enough carbon dioxide (15% of initial) to be objectionable. The polymers that are good barriers to permanent gases, especially oxygen, have important commercial significance. Vinylidene chloride copolymers are available as resins for extrusion, latices for coating, and resins for solvent coating. Vinylidene chloride copolymers are marketed under a variety of trade names. Saran is a trademark of The Dow Chemical Company for vinylidene chloride copolymers. Other trade names include Daran (W.R. Grace), Amsco Res (Union Oil), and Serfene (Morton Chemical) in the United States; and Haloflex (Imperial Chemical Industries, Ltd.), Diofan (BASF), Ixan (Solvay and Cie SA), and Polyidene (Scott-Bader) in Europe. Hydrolyzed ethylene–vinyl acetate copolymers, commonly known as ethylene–vinyl alcohol (EVOH) copolymers, are usually used as extrusion resins, although some may be used in solvent-coating applications. Copolymers of acrylonitrile are used in extrusion and molding applications. Commercially important comonomers for barrier applications include styrene and methyl acrylate. Polyamide polymers can provide a good-to-moderate barrier-to-permeation by permanent gases. Two often-used polymers have adequate properties for some applications. Poly(ethylene terephthalate) is used to make films and bottles. Poly(vinyl chloride) (PVC) is a moderate barrier-to-permanent gases. Plasticized (PVC) is used as a household wrapping film. In regard to water–vapor transmission rate (WVTR) values, those polymers that are good oxygen barriers are often poor water–vapor barriers and vice versa. Polymer molecules without dipole–dipole interactions, such as polyolefins, dissolve very little water and have low WVTR and permeability values. The permeation of flavor, aroma, and solvent molecules in polymers follows the same physics as the permeation of small molecules, but with two significant differences. For these larger molecules, the diffusion coefficients are much lower and the solubility coefficients are much higher. Furthermore, the large solubility coefficient can lead to enough sorption of the large molecule so that plasticization occurs in the polymer, which can increase the diffusion coefficient. Generally, vinylidene chloride copolymers and glassy polymers such as polyamides and EVOH are good barriers to flavor and aroma permeation, whereas the polyolefins are poor barriers. Several physical factors can affect the barrier properties of a polymer. These include temperature, humidity, orientation, and cross-linking. Typically, the permeability increases 5–10% for every increase of 1°C. When a polymer equilibrates with a humid environment, it absorbs water. This can plasticize the polymer and increase the permeability. The effect of orientation on the permeability of polymers is difficult to assess; diffusion in some polymers is unaffected by orientation; in others, increases or decreases are observed. Cross-linking has been shown in a few cases to decrease the diffusion coefficient. Reasonable prediction can be made of the permeabilities of low molecular weight gases such as oxygen, nitrogen, and carbon dioxide in many polymers. The diffusion coefficients are not complicated by the shape of the permeant, and the solubility coefficients of each of these molecules do not vary much from polymer to polymer. Reasonable predictions of the permeabilities of larger molecules such as flavors, aromas, and solvents are not easily made. The diffusion coefficients are complicated by the shape of the permeant, and the solubility coefficients for a specific permeant can vary widely from polymer to polymer. The permachor method is an empirical method for predicting the permeabilities of oxygen, nitrogen, and carbon dioxide in polymers. In this method, a numerical value is assigned to each constituent part of the polymer. An average number is derived for the polymer, and a simple equation converts the value into a permeability. The model has been modified to liquid permeation with some success. For larger molecules, independent predictions of the diffusion coefficients and the solubility coefficients are required. Predicting the diffusion coefficient for a permeant in a polymer requires knowing one other diffusion coefficient in the polymer. The solubility coefficients are more difficult to predict. Although advances are being made, the best method is probably to use a few known solubility coefficients in the polymer to predict others. Measuring the barrier properties of polymers is important for several reasons. The effects of formulation or process changes need to be known, new polymers need to be evaluated, data are needed for a new application before a large investment has been made, and fabricated products need to have performance verified. Two methods of measuring WVTR are commonly used. The newer method uses a Permatran-W (Modern Controls, Inc.). The other method is the American Society for Testing and Materials (ASTM) cup method. Measuring the permeation of carbon dioxide occurs far less often than measuring the permeation of oxygen or water. The simplest method uses the Permatran-C instrument (Modern Controls, Inc.). Many methods are used to characterize the transport of flavor, aroma, and solvent molecules in polymers. Each has some value, and no one method is suitable for all situations. Any experiment should obtain the permeability, the diffusion coefficient, and the solubility coefficient. The primary application for barrier polymers is food and beverage packaging. Barrier polymers are also used for packaging medical products, agricultural products, cosmetics, and electronic components and in moldings, pipe, and tubing. The use of safe materials is vital for barrier applications, particularly for food, medical, and cosmetics packaging. Suppliers of specific barrier polymers can provide the necessary details to ensure safe processing and use of barrier polymers.
