Abstract
Beryllium carbide, Be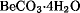 , has been prepared by passing carbon dioxide through an aqueous suspension of beryllium hydroxide. The solid beryllium oxide carbonate intermediates are obtained by a laboratory procedure for preparing pure beryllium salt solutions by reaction with aqueous mineral or organic acids. The beryllium salts of organic acids can be divided into normal carboxylates, Be(RCOO)
, has been prepared by passing carbon dioxide through an aqueous suspension of beryllium hydroxide. The solid beryllium oxide carbonate intermediates are obtained by a laboratory procedure for preparing pure beryllium salt solutions by reaction with aqueous mineral or organic acids. The beryllium salts of organic acids can be divided into normal carboxylates, Be(RCOO).gif) , is prepared by crystallization from a solution of beryllium hydroxide or beryllium oxide carbonate in a slight excess of dilute nitric acid. Beryllium nitride, Be
, is prepared by crystallization from a solution of beryllium hydroxide or beryllium oxide carbonate in a slight excess of dilute nitric acid. Beryllium nitride, Be.gif) , is obtained by evaporating a solution of beryllium hydroxide or oxide carbonate in a slight excess of oxalic acid. Beryllium oxalate is important for the preparation of ultrapure beryllium hydroxide, used as a standard for spectrographic analysis of beryllium compounds. Beryllium oxide, BeO, is the most important high purity commercial beryllium chemical. High purity beryllium sulfate,
, is obtained by evaporating a solution of beryllium hydroxide or oxide carbonate in a slight excess of oxalic acid. Beryllium oxalate is important for the preparation of ultrapure beryllium hydroxide, used as a standard for spectrographic analysis of beryllium compounds. Beryllium oxide, BeO, is the most important high purity commercial beryllium chemical. High purity beryllium sulfate, .gif) is calcined at carefully controlled temperatures selected to give tailored properties of the beryllium oxide powders as required by the individual beryllia ceramic fabricators. High purity beryllium oxide powder is fabricated by classical ceramic-forming processes such as dry pressing, isostatic pressing, extrusion, tape casting, and slip casting. Beryllia ceramics offer the advantages of a unique combination of high thermal conductivity and heat capacity with high electrical resistivity. Beryllium is principally consumed in the metallic form, either as an alloy constituent or as the pure metal. Consequently, there is no industry associated with beryllium compounds except for beryllium oxide, BeO, which is commercially important as a ceramic material. Care must be taken in the fabrication and processing of beryllium products to avoid inhalation of airborne beryllium particulate matter such as dusts, mists, or fumes in excess of prescribed work place limits. Inhalation of fine airborne beryllium may cause chronic beryllium disease, a serious lung disorder, in certain sensitive individuals.
is calcined at carefully controlled temperatures selected to give tailored properties of the beryllium oxide powders as required by the individual beryllia ceramic fabricators. High purity beryllium oxide powder is fabricated by classical ceramic-forming processes such as dry pressing, isostatic pressing, extrusion, tape casting, and slip casting. Beryllia ceramics offer the advantages of a unique combination of high thermal conductivity and heat capacity with high electrical resistivity. Beryllium is principally consumed in the metallic form, either as an alloy constituent or as the pure metal. Consequently, there is no industry associated with beryllium compounds except for beryllium oxide, BeO, which is commercially important as a ceramic material. Care must be taken in the fabrication and processing of beryllium products to avoid inhalation of airborne beryllium particulate matter such as dusts, mists, or fumes in excess of prescribed work place limits. Inhalation of fine airborne beryllium may cause chronic beryllium disease, a serious lung disorder, in certain sensitive individuals.
