Abstract
Caprolactam (2-oxohexamethylenimine, hexahydro-2H-azepin-2-one) is one of the most widely used chemical intermediates. However, almost all of the annual production of 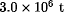 is consumed as the monomer for nylon-6 fibers and plastics. Cyclohexanone, which is the most common organic precursor of caprolactam, is made from benzene by either phenol hydrogenation or cyclohexane oxidation. Caprolactam is a white, hygroscopic, crystalline solid at ambient temperature, with a characteristic odor. It is very soluble in water and in most common organic solvents and is sparingly soluble in high molecular weight aliphatic hydrocarbons. Molten caprolactam is a powerful solvent for polar and nonpolar organic chemicals. The low melting point of caprolactam and its stability and low viscosity form the basis for commercial transportation as a liquid in insulated tank cars or trucks. Caprolactam is an amide and therefore undergoes the reactions of this class of compounds. It can be hydrolyzed, N-alkylated, O-alkylated, nitrosated, halogenated, and subjected to many other reactions. All commercial processes for the manufacture of caprolactam are based on either toluene or benzene, each of which occurs in refinery BTX-extract streams. Alkylation of benzene with propylene yields cumene, a source of phenol; ca 10% of U.S. phenol is converted to caprolactam. Worldwide caprolactam demand increased by about 2% per year during the 1980s, and similar growth is expected into the 1990s. DSM America produces caprolactam only for merchant sales, both domestic and foreign. BASF is a customer, following acquisition of Enka's U.S. fiber and plastics plants, and also a captive producer of caprolactam. Allied-Signal's production is primarily captive for nylon-6 fibers and plastics, but substantial amounts are supplied to the export market. Caprolactam has a low order of toxicity. It presents no appreciable health hazard if it is handled properly. Prolonged exposure to dust or vapors causes irritation of eyes, mucous membranes, and skin; inhalation may cause irritation of the respiratory tissues. Skin contact, if prolonged, can lead to dermatosis. A thorough wash with water, in which caprolactam is very soluble, or with soap and water, normally is sufficient to remove caprolactam from contaminated parts of the body. Caprolactam has been extensively tested for its mutagenic potential. Based on the overall weight of evidence, caprolactam would be considered nonmutagenic.
is consumed as the monomer for nylon-6 fibers and plastics. Cyclohexanone, which is the most common organic precursor of caprolactam, is made from benzene by either phenol hydrogenation or cyclohexane oxidation. Caprolactam is a white, hygroscopic, crystalline solid at ambient temperature, with a characteristic odor. It is very soluble in water and in most common organic solvents and is sparingly soluble in high molecular weight aliphatic hydrocarbons. Molten caprolactam is a powerful solvent for polar and nonpolar organic chemicals. The low melting point of caprolactam and its stability and low viscosity form the basis for commercial transportation as a liquid in insulated tank cars or trucks. Caprolactam is an amide and therefore undergoes the reactions of this class of compounds. It can be hydrolyzed, N-alkylated, O-alkylated, nitrosated, halogenated, and subjected to many other reactions. All commercial processes for the manufacture of caprolactam are based on either toluene or benzene, each of which occurs in refinery BTX-extract streams. Alkylation of benzene with propylene yields cumene, a source of phenol; ca 10% of U.S. phenol is converted to caprolactam. Worldwide caprolactam demand increased by about 2% per year during the 1980s, and similar growth is expected into the 1990s. DSM America produces caprolactam only for merchant sales, both domestic and foreign. BASF is a customer, following acquisition of Enka's U.S. fiber and plastics plants, and also a captive producer of caprolactam. Allied-Signal's production is primarily captive for nylon-6 fibers and plastics, but substantial amounts are supplied to the export market. Caprolactam has a low order of toxicity. It presents no appreciable health hazard if it is handled properly. Prolonged exposure to dust or vapors causes irritation of eyes, mucous membranes, and skin; inhalation may cause irritation of the respiratory tissues. Skin contact, if prolonged, can lead to dermatosis. A thorough wash with water, in which caprolactam is very soluble, or with soap and water, normally is sufficient to remove caprolactam from contaminated parts of the body. Caprolactam has been extensively tested for its mutagenic potential. Based on the overall weight of evidence, caprolactam would be considered nonmutagenic.
Keywords: Caprolactam; Nylon; Allied-Signal process; Toluene; Benzene; Stamicarbon; Cyclohexanone; BTX-extract; BASF
