Abstract
Organic compounds are nitrated by both ionic and free-radical reactions. Ionic nitrations are employed extensively with aromatics, compounds containing hydroxy groups (such as alcohols, glycerol, and cellulose), and amines. Nitronium ions (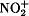 ) and sometimes nitrosonium ions (NO+) attack the above compounds to start the nitration reactions. Nitronium ions are present in most nitrating agents employed, including mixtures of HNO3 and H2SO4; mixtures of N2O4 and H2SO4; HNO3; and N2O5. The chemistry and physical steps during nitrations that involve two liquid phases are discussed. Key features of several important commercial nitrations are described, which in some cases exhibit extremely rapid rates of nitration. Considerable effort has been made to develop commercial processes with improved safety of operation, reduced environmental problems, lesser energy demands, lower operating costs, and increased yields of nitrated products. Certain nitrated products can only be produced using N2O5 as a nitrating agent. Some N2O5 processes likely will be commercialized in the near future for the production of high explosives and nitramines. Paraffins and cycloparaffins react with HNO3 or NO2 via free-radical steps to form nitroparaffins. The chemistry and processes are discussed. Vol. 17, pp. 68–80, 39 refs. to June 1993.
) and sometimes nitrosonium ions (NO+) attack the above compounds to start the nitration reactions. Nitronium ions are present in most nitrating agents employed, including mixtures of HNO3 and H2SO4; mixtures of N2O4 and H2SO4; HNO3; and N2O5. The chemistry and physical steps during nitrations that involve two liquid phases are discussed. Key features of several important commercial nitrations are described, which in some cases exhibit extremely rapid rates of nitration. Considerable effort has been made to develop commercial processes with improved safety of operation, reduced environmental problems, lesser energy demands, lower operating costs, and increased yields of nitrated products. Certain nitrated products can only be produced using N2O5 as a nitrating agent. Some N2O5 processes likely will be commercialized in the near future for the production of high explosives and nitramines. Paraffins and cycloparaffins react with HNO3 or NO2 via free-radical steps to form nitroparaffins. The chemistry and processes are discussed. Vol. 17, pp. 68–80, 39 refs. to June 1993.
Keywords: Ionic nitration; Aromatic nitration; Kinetics; Free radicals; Nitration; Paraffins; Explosives
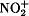 ) and sometimes nitrosonium ions (NO
) and sometimes nitrosonium ions (NO