Abstract
Oligosaccharide antibiotics represented by the everninomicins, eg, everninomicin B (C66H99Cl2NO36) and everninomicin C (C63H93Cl2NO34); flambamycins, eg, flambamycin (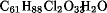 ), avilamycins, eg, avilamycin A (C61H88Cl2O32) and avilamycin B (C59H84Cl2O32); and curamycins, eg, curamycin A (C59H84Cl2O32), have complex and unique structural features. They are sometimes referred to as orthosomycins because characteristically these oligosaccharides possess two acid-sensitive ortho ester linkages, cleavage of which results in the complete loss of antibiotic activity. Another structural feature common to all the oligosaccharide antibiotics is the presence of a substituted phenol ester derived from dichloroisoeverninic acid attached to the sugar ring B at C-13, essential for the antibiotic activity. Oligosaccharide antibiotics are colorless solids, are often crystalline, and have defined melting points and optical rotations. They are sensitive to acid pH because of the ortho ester linkages. Yet all of them have an acidic phenolic group which makes the molecule relatively unstable to handling conditions. Everninomicin D is the principal component from cultures of Micromonospora carbonacae. Chemical modification of the nitro group in everninomicin D has resulted in the formation of amino, mono- and dialkylamino, N-acylamino, and N-hydroxylamino (and its nitrone derivatives) everninomicin D, all of which possess great antibiotic activity against gram-positive bacteria. Everninomicin B, a secondary component, has been extensively investigated because of its improved pharmacokinetic properties. Flambamycin, produced by Streptomyces hygroscopicus DS 23230, also has undergone substantial chemical degradative experiments during structure elucidation. The structure, chemical reactions, and biological properties have been thoroughly reviewed. Curamycin A is the primary component of the culture Streptomyces curacoi. At least 16 avilamycins have been reported. Sporacuracin A, C63H94Cl2O35, mp 145–148°C, []D
), avilamycins, eg, avilamycin A (C61H88Cl2O32) and avilamycin B (C59H84Cl2O32); and curamycins, eg, curamycin A (C59H84Cl2O32), have complex and unique structural features. They are sometimes referred to as orthosomycins because characteristically these oligosaccharides possess two acid-sensitive ortho ester linkages, cleavage of which results in the complete loss of antibiotic activity. Another structural feature common to all the oligosaccharide antibiotics is the presence of a substituted phenol ester derived from dichloroisoeverninic acid attached to the sugar ring B at C-13, essential for the antibiotic activity. Oligosaccharide antibiotics are colorless solids, are often crystalline, and have defined melting points and optical rotations. They are sensitive to acid pH because of the ortho ester linkages. Yet all of them have an acidic phenolic group which makes the molecule relatively unstable to handling conditions. Everninomicin D is the principal component from cultures of Micromonospora carbonacae. Chemical modification of the nitro group in everninomicin D has resulted in the formation of amino, mono- and dialkylamino, N-acylamino, and N-hydroxylamino (and its nitrone derivatives) everninomicin D, all of which possess great antibiotic activity against gram-positive bacteria. Everninomicin B, a secondary component, has been extensively investigated because of its improved pharmacokinetic properties. Flambamycin, produced by Streptomyces hygroscopicus DS 23230, also has undergone substantial chemical degradative experiments during structure elucidation. The structure, chemical reactions, and biological properties have been thoroughly reviewed. Curamycin A is the primary component of the culture Streptomyces curacoi. At least 16 avilamycins have been reported. Sporacuracin A, C63H94Cl2O35, mp 145–148°C, []D .gif) , and sporacuracin B, C63H94Cl2O35, mp 162–165°C, []D
, and sporacuracin B, C63H94Cl2O35, mp 162–165°C, []D .gif) , are other probable members of the oligosaccharide antibiotic family. Oligosaccharides in general are highly potent, but are narrow-spectrum antibiotics. Everninomicins are active against a wide variety of gram-positive aerobes and anaerobes; Neisseria, Mycoplasma, and some Mycobacteria. Potential for oligosaccharide antibiotics in both human and animal health care has been claimed.
, are other probable members of the oligosaccharide antibiotic family. Oligosaccharides in general are highly potent, but are narrow-spectrum antibiotics. Everninomicins are active against a wide variety of gram-positive aerobes and anaerobes; Neisseria, Mycoplasma, and some Mycobacteria. Potential for oligosaccharide antibiotics in both human and animal health care has been claimed.
Keywords: Oligosaccharides; Everninomicins; Flambamycin; Curamycin; Avilamycin; Biological properties
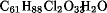 ), avilamycins, eg, avilamycin A (C
), avilamycins, eg, avilamycin A (C.gif) , and sporacuracin B, C
, and sporacuracin B, C.gif) , are other probable members of the oligosaccharide antibiotic family. Oligosaccharides in general are highly potent, but are narrow-spectrum antibiotics. Everninomicins are active against a wide variety of gram-positive aerobes and anaerobes; Neisseria, Mycoplasma, and some Mycobacteria. Potential for oligosaccharide antibiotics in both human and animal health care has been claimed.
, are other probable members of the oligosaccharide antibiotic family. Oligosaccharides in general are highly potent, but are narrow-spectrum antibiotics. Everninomicins are active against a wide variety of gram-positive aerobes and anaerobes; Neisseria, Mycoplasma, and some Mycobacteria. Potential for oligosaccharide antibiotics in both human and animal health care has been claimed.