Abstract
The proliferation of portable electronic devices has fueled rapid market growth for the rechargeable battery industry. Miniaturization of electronics coupled with consumer demand for lightweight batteries providing ever longer run times continues to spur interest in advanced battery systems. Interest also continues to run strong in electric vehicles (EVs) and the large auto manufacturers continue to develop prototype EVs. Advanced batteries continue to play a strong role in other applications such as load leveling for the electric utility industry and satellite power systems for aerospace. Secondary battery systems have been based on aqueous electrolytes. The use of water imposes a fundamental limitation on battery voltage because of the electrolysis of water. The application of nonaqueous electrolytes affords a significant advantage in terms of achievable battery voltages. By far the most actively researched field in nonaqueous battery systems has been the development of practical rechargeable lithium batteries based on the use of lithium metal, Li, or a lithium alloy, as the negative electrode. The use of lithium as a negative electrode for secondary batteries offers a number of advantages. Lithium has the lowest equivalent weight of any metal and affords very negative electrode potentials when in equilibrium with solvated lithium ions, resulting in very high theoretical energy densities for battery couples. These high theoretical energy densities have prompted a wealth of research activity in a wide variety of experimental battery systems. However, realization of the technology to commercialize these systems has been slow. A key technical problem in developing practical lithium batteries has been poor cycle life attributable to the lithium electrode. The highly reactive nature of freshly plated lithium leads to reactions with electrolyte and impurities to form passivating films that electrically isolate the lithium metal. The formation of surface films on the lithium electrode imparts the apparent stability of the electrolyte to the electrode. In addition to providing a stable film in the presence of lithium, the electrolyte must satisfy additional requirements, including good conductivity, being in the liquid range over the battery operating temperature, and electrochemical stability over a wide voltage range. In order to satisfy the various electrolyte system requirements, the use of mixed solvent electrolytes has become common in practical cells. Examples are tetrahydrofuran, C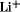 ion into a host lattice. Intercalation electrodes have found wide application in systems employing both solid or liquid electrolytes. The use of high temperature lithium cells for electric vehicle applications has been under development since the 1970s. Advances in the development of lithium alloy–metal sulfide batteries have led to lithium–aluminum/metal sulfide batteries, ie, the Li–Al/FeS system. The cell employs a molten salt electrolyte, most commonly a lithium chloride/potassium chloride, LiCl–KCl eutectic mixture. The negative electrode is composed of lithium–aluminum alloy, which operates at about 300 mV positive of pure lithium. The positive electrode is composed of iron sulfide mixed with a conductive agent such as carbon or graphite. Electrodes are constructed by cold pressing powder onto current collectors. The best known of the high temperature batteries is the sodium–sulfur, Na–S, battery. The cell is constructed using a solid electrolyte typically consisting of -alumina, -Al
ion into a host lattice. Intercalation electrodes have found wide application in systems employing both solid or liquid electrolytes. The use of high temperature lithium cells for electric vehicle applications has been under development since the 1970s. Advances in the development of lithium alloy–metal sulfide batteries have led to lithium–aluminum/metal sulfide batteries, ie, the Li–Al/FeS system. The cell employs a molten salt electrolyte, most commonly a lithium chloride/potassium chloride, LiCl–KCl eutectic mixture. The negative electrode is composed of lithium–aluminum alloy, which operates at about 300 mV positive of pure lithium. The positive electrode is composed of iron sulfide mixed with a conductive agent such as carbon or graphite. Electrodes are constructed by cold pressing powder onto current collectors. The best known of the high temperature batteries is the sodium–sulfur, Na–S, battery. The cell is constructed using a solid electrolyte typically consisting of -alumina, -Al
Keywords: Ambient temperature lithium systems; Solid electrolyte systems; Coin system; Button cell; High temperature systems; Aluminum cells; Sodium-sulfur
