Abstract
Rhenium is one of the least abundant elements, existing in nature only in trace quantities. The commercial source of rhenium is in molybdenite by-product material from porphyry copper ores. Rhenium oxides are driven off upon roasting the molybdenite concentrate. Rhenium is separated, converted to an ammonium salt, and reduced to the metal.
The metal has found many high temperature uses, both as pure metal and as alloys with elements such as molybdenum, tungsten, and nickel. Uses include filaments in a wide array of bulbs and mass spectrometers, in thermocouples, and in heating elements. It is also used as a catalyst in the production of gasolines. Rhenium-containing nickel-based alloys are used for aircraft turbine engines.
Rhenium forms a wide variety of compounds in oxidation states 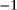 to
to .gif) . High oxidation state compounds are common; low oxidation state compounds are restricted primarily to metal carbonyls and derivatives. Polynuclear compounds having strong metal–metal bonds are plentiful. Little is known concerning the toxicity of rhenium or its compounds.
. High oxidation state compounds are common; low oxidation state compounds are restricted primarily to metal carbonyls and derivatives. Polynuclear compounds having strong metal–metal bonds are plentiful. Little is known concerning the toxicity of rhenium or its compounds.
Keywords: Occurrence; Properties; Sources; Manufacture; Shipment; Economics; Grades; Analytical Methods; Enviornmental Concerns; Recycling; Health; Alloys; Ammonium Perrhenate; Perrhenic Acid; Rhenium Carbonyls; Rhenium Oxides; Rhenium Sulfides; Rhenium Halides; Rhenium Hydrides; Air Craft Turbine Engines; Catalysts; Petroleum
